Biden administration fights attempts to block abortion medication
The Department of Justice appealed a federal judge’s ruling that the FDA had illegally approved mifepristone 23 years ago.
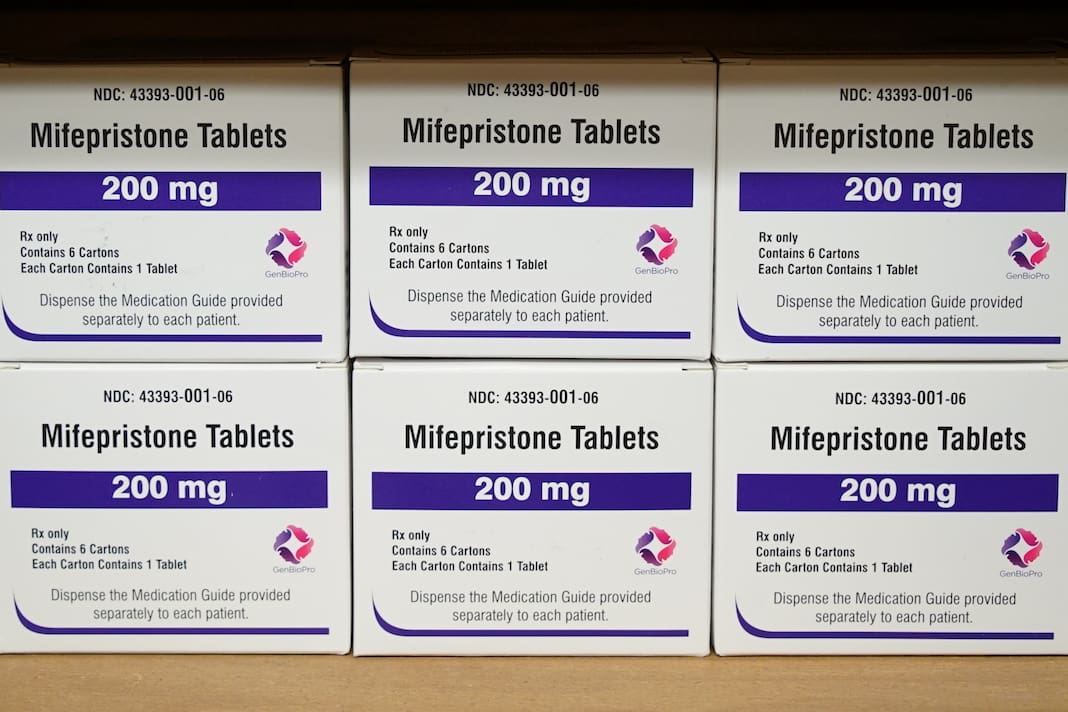
On Monday, the Justice Department filed a request to block an unprecedented ruling by a federal judge in Texas to suspend the Food and Drug Administration’s approval of an abortion medication.
“My Administration will fight this ruling,” President Joe Biden said in a statement. “Vice President Harris and I will continue to lead the fight to protect a woman’s right to an abortion, and to make her own decisions about her own health. That is our commitment.”
On April 7, Texas U.S. District Court Judge Matthew Kacsmaryk, who was appointed by former President Donald Trump, suspended the FDA’s 23-year-old approval of the drug mifepristone, claiming that the original approval had been unlawful.
“The error in FDA’s judgment is borne out by myriad stories and studies brought to the Court’s attention,” Kacsmaryk wrote, adding that “a preliminary injunction would serve the public interest.”
The ruling pauses its applicability for seven days to allow the government time to appeal.
The Justice Department and the manufacturer of mifepristone petitioned the 5th U.S. Circuit Court of Appeals to place both short- and long-term stays on Kacsmaryk’s ruling while the case winds its way through the appeals process.
On the same day Kacsmaryk ruled, and in stark contrast, Washington state U.S. District Court Judge Thomas Rice, appointed by former President Barack Obama, ordered the FDA not to restrict access to the abortion medication in the 17 Democratic-led states and the District of Columbia where attorney generals had sued to keep mifepristone available.
Mifepristone is used in over half the abortions carried out in the U.S. It’s part of a two-drug procedure in which a patient takes mifepristone, then takes a second drug called misoprostol. Medication abortion is a preferred method of abortion for many people because it can be both prescribed by telehealth and taken at home rather than in a clinic.
A staunch conservative, Kacsmaryk in his ruling referred to doctors who perform reproductive health services as “abortionists” and to a fetus as an “unborn child” or “unborn human” — language commonly used by anti-abortion activists.
In a statement issued by the Department of Justice after Kacsmaryk’s ruling, Attorney General Merrick Garland said: “Today’s decision overturns the FDA’s expert judgment, rendered over two decades ago, that mifepristone is safe and effective. The Department will continue to defend the FDA’s decision. … The Department is committed to protecting Americans’ access to legal reproductive care.”
In an appearance on MSNBC Sunday, Health and Human Services Secretary Xavier Becerra told host Jonathan Capehart, “We will seek a stay of that judge’s ruling beyond the seven days that the judge imposed his own stay, and we will make sure that we get that appeal, the stay, and if we can’t get the stay, we will go as far as we need to go to try to protect access to mifepristone.”
In a statement to The Hill, a spokesperson for the FDA explained that the agency stood by its approval, saying that the abortion medication was approved “based on a comprehensive review of the scientific evidence available. …The approval was based on the best available science and done in accordance with the laws that govern our work.”
According to the Associated Press, Jennifer Dalven, director of the Reproductive Freedom Project at the American Civil Liberties Union, said Monday that providers are essentially on hold until this Friday, when the courts decide next steps: “We don’t know exactly what will happen. … What we do know is that there will be significant confusion and chaos as providers try to provide the best care they possibly can for their patients.”
As writers for Slate explained in a report in February, the Supreme Court ruled in 1985 that the Food and Drug Administration has discretion in the enforcement of statutes regarding the approval of drugs. Kacsmaryk therefore doesn’t have the legal authority to force the FDA to remove a drug from the market; he can only force the agency to go through the process of congressional hearings and deliberation on the legality of the approval.
Kacsmaryk’s order only applies to the companies named in the lawsuit: Danco, the brand manufacturer of mifepristone, and GenBioPro, the maker of the generic version of the drug. He would not be able to unilaterally stop distribution of those drugs without allowing both the manufacturers and physicians due process in court to appeal.
Over 300 pharmaceutical company executives signed an open letter posted Monday in support of the FDA, calling for the reversal of the judge’s ruling on mifepristone.
“If courts can overturn drug approvals without regard for science or evidence, or for the complexity required to fully vet the safety and efficacy of new drugs, any medicine is at risk for the same outcome as mifepristone,” the letter reads.
Published with permission of The American Independent Foundation.
Recommended

Biden campaign launches new ad focused on Affordable Care Act
Former President Trump has said he wants to do away with the popular health care law.
By Kim Lyons, Pennsylvania Capital-Star - May 08, 2024
Ohio doctors fear effects of emergency abortion care case set to go before U.S. Supreme Court
A federal law that allows emergency departments to treat patients without regard to their ability to pay will be under U.S. Supreme Court scrutiny this week, and Ohio doctors are concerned about the case’s local impact on emergency abortion care.
By Susan Tebben, Ohio Capital Journal - April 23, 2024
House GOP votes to end flu, whooping cough vaccine rules for foster and adoptive families
A bill to eliminate flu and whooping cough vaccine requirements for adoptive and foster families caring for babies and medically fragile kids is heading to the governor’s desk.
By Anita Wadhwani, Tennessee Lookout - March 26, 2024









































































